Most people assume that if a drug is available as a generic, it’s just as good as the brand name-and for most medications, that’s true. But there are real, medically valid reasons why your doctor might write a prescription that says "dispense as written" or "brand medically necessary". It’s not about preference. It’s not about profit. Sometimes, it’s about keeping you safe.
When Generics Aren’t Enough
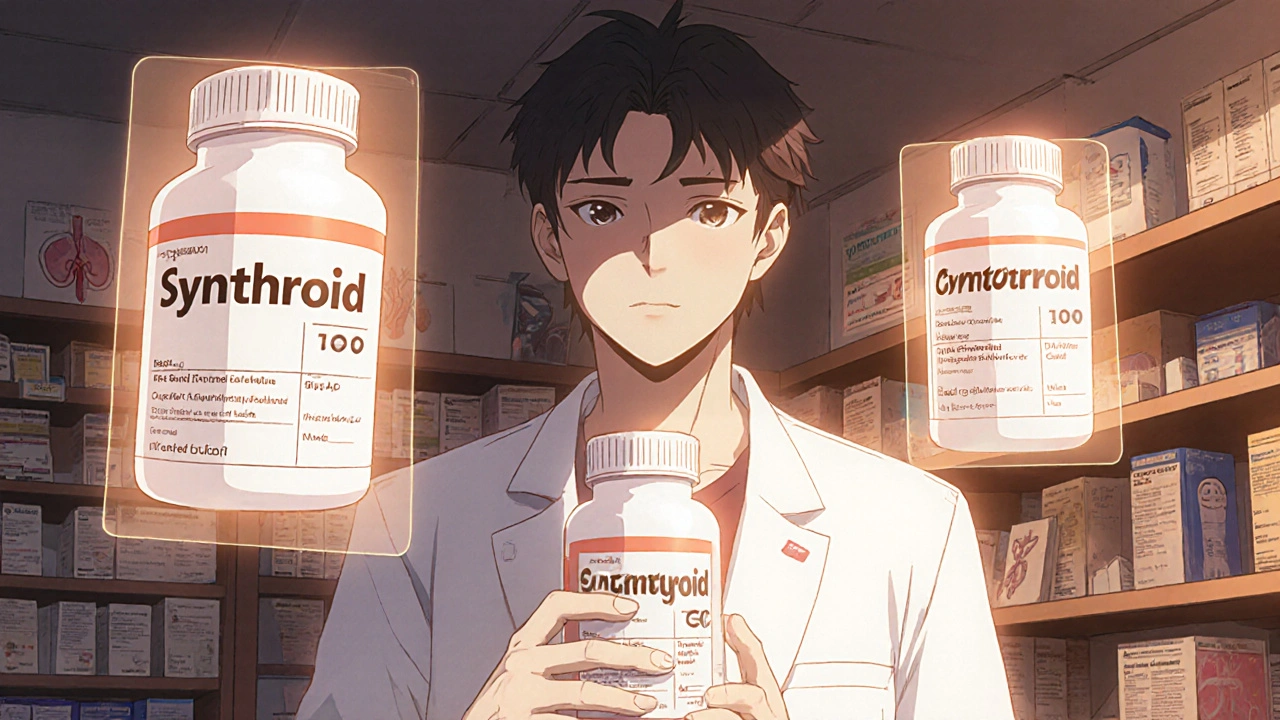
Generic drugs are required by the FDA to be bioequivalent to their brand-name counterparts. That means they must deliver the same active ingredient at the same rate and amount into your bloodstream. The acceptable range? Between 80% and 125% of the brand’s performance. Sounds close enough, right? But for some drugs, even a 20% variation can be dangerous. These are called narrow therapeutic index (NTI) drugs. With NTIs, the difference between a therapeutic dose and a toxic one is razor-thin. A little too much, and you risk serious side effects. A little too little, and the treatment fails. Examples include:- Levothyroxine (Synthroid) for thyroid disorders
- Warfarin (Coumadin) for blood thinning
- Levetiracetam (Keppra) for epilepsy
- Cyclosporine for organ transplant patients
It’s Not Just About the Active Ingredient
Generics have the same active ingredient, yes. But they can contain different fillers, dyes, coatings, and binders. These are called inactive ingredients. For most people, they don’t matter. But for some, they do. Take ciprofloxacin, an antibiotic. One generic version might use a different coating that irritates the stomach. Another might contain lactose-problematic for someone with severe intolerance. Patients have reported nausea, bloating, or even diarrhea after switching to a different generic manufacturer, even though the active drug was identical. This isn’t rare. A 2022 analysis of patient complaints on Drugs.com found that 37% of negative reviews for generic antibiotics mentioned inconsistent side effects tied to inactive ingredients. These aren’t allergies. They’re intolerances. And they’re real. Some brand-name drugs also use proprietary delivery systems. Advair’s Diskus inhaler, for example, is designed to release medication in a very specific way. Generic versions may contain the same drugs, but the delivery mechanism can differ. For someone with severe asthma, that difference can mean the difference between relief and an emergency room visit.Why Doctors Still Prescribe Brand Names
Doctors aren’t prescribing brand names because they’re paid to. In fact, most don’t get any incentive at all. The reasons are more subtle-and often rooted in experience. A 2018 study of over one million prescription notes found that doctors used brand names in 15-20% of cases, even when generics were available. Why? Because they’ve seen what happens when patients switch. One patient with epilepsy had a seizure after a generic swap. Another with hypothyroidism developed heart palpitations. These aren’t theoretical risks. They’re lived experiences. The American Medical Association’s guidelines are clear: brand-name prescribing is only justified when scientific evidence shows a real difference in outcomes. That means:- NTI drugs
- Patients who’ve had documented failure with multiple generics
- Patients with confirmed reactions to inactive ingredients
Insurance and the Real Cost
The cost difference is staggering. In 2022, the average brand-name prescription cost $471.67. The generic? $13.76. That’s over 97% less. If your doctor prescribes a brand-name drug without a valid medical reason, your insurance might not cover it-or they’ll make you pay a much higher copay. One Kaiser Family Foundation survey found that 42% of patients paid more out of pocket simply because their doctor prescribed a brand name when a generic was available. Insurance companies know this. When a doctor writes “brand medically necessary,” they often require prior authorization. That means your doctor has to submit paperwork, and you might wait up to 72 hours for approval. Approval rates vary: 89% for epilepsy drugs, but only 45% for acid reflux medications like omeprazole. If your doctor prescribes a brand name and your insurance denies it, you’re left with two choices: pay hundreds of dollars, or switch to a generic. And if you’ve already had a bad reaction? That’s not a choice-it’s a risk.What You Can Do
You don’t have to accept a brand-name prescription without asking questions. Here’s what to do:- Ask: “Is there a generic version available?”
- If yes: “Is this one of those drugs where switching could be risky?”
- If you’ve had a bad reaction to a generic before: Tell your doctor. Write it down. Ask them to note it in your chart.
- If your doctor insists on brand: Ask them to write “dispense as written” or “brand medically necessary” on the prescription. This prevents the pharmacist from substituting.
- Check the FDA’s Orange Book. It lists which generics are rated as equivalent. You can ask your pharmacist for this info.

What’s Changing
The FDA is paying more attention. In 2023, new guidance required generic manufacturers to match the shape and color of brand-name pills to reduce confusion and errors. That’s a big step-because many medication mistakes happen when patients think they got the wrong drug because it looks different. There’s also a growing trend called “authorized generics.” These are made by the original brand company but sold under a generic label. They’re identical to the brand in every way-active ingredient, inactive ingredients, even the coating. They’re cheaper than the brand but avoid the variability between generic manufacturers. And in the future, biosimilars (generic versions of biologic drugs) will become more common. These are complex molecules, and switching them carries even higher risks. Experts predict that by 2027, 45-60% of biologic prescriptions will be filled with biosimilars-but only if patients are carefully monitored.The Bottom Line
For 90% of prescriptions, generics are just as safe and effective. They save billions every year. That’s a good thing. But for a small group of patients-those on NTI drugs, those with known sensitivities, or those who’ve had bad reactions-brand-name drugs aren’t a luxury. They’re a necessity. The key is knowing which side you’re on. If you’re taking a medication where small changes matter, don’t assume generics are interchangeable. Talk to your doctor. Ask questions. Know your options. And never be afraid to speak up if something feels off after a switch.Can pharmacists substitute a generic if the doctor wrote "brand medically necessary"?
No. If a doctor writes "dispense as written," "do not substitute," or "brand medically necessary" on the prescription, pharmacists are legally required to fill it exactly as written. In 49 U.S. states and Washington D.C., pharmacists can substitute generics only if the prescriber doesn’t block it. Texas has additional rules for certain drugs, but the principle is the same: the prescriber’s instruction overrides automatic substitution.
Why do some patients react badly to generics even though they’re "the same"?
Generics contain the same active ingredient, but they can use different inactive ingredients-like fillers, dyes, or coatings. These can affect how quickly the drug dissolves or how your body absorbs it. For people with sensitivities, these differences can cause nausea, bloating, or even changes in how well the drug works. This is especially true for narrow therapeutic index drugs, where small changes in blood levels can cause serious problems.
Are brand-name drugs better quality than generics?
No. The FDA holds generic manufacturers to the same quality standards as brand-name companies. Both must meet strict rules for purity, strength, and manufacturing. The difference isn’t quality-it’s consistency. Brand-name drugs are made by one company, so every batch is identical. Generics may come from different manufacturers, and while each batch meets FDA standards, small variations between manufacturers can occur.
How do I know if my drug has a narrow therapeutic index?
Common NTI drugs include levothyroxine, warfarin, phenytoin, cyclosporine, and digoxin. Your doctor or pharmacist should know. You can also check the FDA’s Orange Book online or ask your pharmacist to look it up. If you’re on one of these drugs, it’s wise to stick with the same brand or manufacturer unless your doctor advises otherwise.
What should I do if my insurance denies coverage for a brand-name drug?
Ask your doctor to file a prior authorization request. They’ll need to explain why the brand is medically necessary-often using the FDA’s list of NTI drugs or your personal history of adverse reactions. If denied, ask if an authorized generic is available. These are made by the brand company and often covered by insurance at a lower cost. If all else fails, ask about patient assistance programs from the drug manufacturer.
Is it true that brand-name drugs help fund future innovation?
It’s a common argument from drug companies, but it’s misleading. Most brand-name drugs are already off-patent and no longer fund new research. The profits from these older drugs go to shareholders, not R&D. True innovation comes from new drugs still under patent, which are rarely replaced by generics. Prescribing brand names for old, off-patent drugs doesn’t fund future breakthroughs-it just increases costs for patients and insurers.

Christine Eslinger
November 18, 2025 AT 02:24For anyone on levothyroxine-please, don’t switch generics without telling your doctor. I went from Synthroid to a generic and developed brain fog so bad I forgot my own birthday. Took three months to figure out it was the med. Now I insist on the brand. It’s not expensive compared to missing work or losing your mind.
Also, the FDA’s 80-125% bioequivalence range? That’s wild when you think about it. That’s like saying a 100-degree fever is fine if it’s 80 or 125. But for some drugs, it’s literally life or death.
Doctors aren’t being greedy. They’re just trying to keep you from crashing.
And if your pharmacy switches your pill without telling you? Ask for the manufacturer name. Write it down. Fight for consistency. Your body remembers.
Generics are great. But not when your thyroid’s involved.
PS: The FDA’s new color/shape rules? Long overdue. I once thought I got the wrong pill because it was round instead of oval. Panic attack ensued.
Denny Sucipto
November 18, 2025 AT 03:51Man, I wish I’d known this five years ago. My buddy was on Keppra, switched to generic, had a seizure at his kid’s soccer game. Kid was six. He still blames himself. I told him it wasn’t his fault-but it still gutted him.
Now I make sure my grandma’s thyroid med never changes. She doesn’t even know what a generic is. I just tell the pharmacist: ‘Same one as last time.’ Simple. Done.
And yeah, the cost difference is insane. But if you’re the one who ends up in the ER because a pill looked different? No amount of savings is worth that.
Gabriella Jayne Bosticco
November 19, 2025 AT 14:45Interesting how we treat meds like they’re interchangeable like socks. But your body’s not a washing machine.
I’ve seen people freak out over generic ibuprofen because it’s blue instead of white. Turns out they were just used to the brand’s coating. Weird, but real.
Also, authorized generics? That’s the secret weapon. Same pill, cheaper price. More people should know about that.
Sarah Frey
November 21, 2025 AT 12:36It is imperative to recognize that the pharmacological equivalence of generic medications does not equate to clinical interchangeability in all contexts, particularly with narrow therapeutic index agents. The FDA’s bioequivalence thresholds, while statistically valid, are not physiologically uniform across patient populations. Subtle variations in excipient composition may precipitate clinically significant alterations in pharmacokinetics among susceptible individuals, particularly those with comorbid gastrointestinal or metabolic conditions. Therefore, prescriber discretion, grounded in empirical clinical observation, remains a critical safeguard in therapeutic management.
Furthermore, the assertion that brand-name drugs do not fund innovation is empirically flawed; while many are off-patent, the revenue stream from legacy products enables continued investment in novel drug development across pharmaceutical enterprises. Dismissing this dynamic oversimplifies the economic ecosystem of modern medicine.
Bailey Sheppard
November 21, 2025 AT 23:28I’ve been on warfarin for 12 years. Switched generics once. INR went haywire. Ended up in the hospital with a bruise the size of a grapefruit. Never again.
Now I call my pharmacy every time I refill. ‘Same manufacturer?’ Yes. ‘Good.’
Also, the ‘brand medically necessary’ note? That’s your armor. Use it.
And yeah, I know it’s expensive. But so is a stroke.
Kristi Joy
November 22, 2025 AT 16:25If you’re on one of these meds, don’t feel bad for asking for the brand. You’re not being difficult-you’re being smart.
I used to feel guilty asking my doctor for Synthroid instead of the generic. Like I was being high-maintenance. But then I read about how even tiny changes can mess with your mood, energy, heart rate.
Now I say: ‘I’m not asking for luxury. I’m asking for stability.’
And if your insurance pushes back? That’s not your fault. It’s a broken system. You’re not the problem.
You’re just trying to stay alive.
Hal Nicholas
November 24, 2025 AT 04:52Oh here we go. Another ‘trust your doctor’ lecture. Meanwhile, Big Pharma is laughing all the way to the bank.
Doctors don’t care about your seizures. They care about their quota of prescriptions. They don’t even know what’s in the generic. Most of them think ‘generic’ means ‘cheap.’
And the FDA? They’re just rubber-stamping whatever Big Pharma pays them to approve.
They let you switch to a generic that’s 20% weaker? That’s not science. That’s corporate greed dressed up as regulation.
And don’t even get me started on authorized generics. That’s just the brand company milking the system again.
Wake up. You’re being played.
Louie Amour
November 24, 2025 AT 08:43Let me guess-you’re one of those people who thinks ‘FDA approved’ means ‘safe.’
Newsflash: The FDA doesn’t test drugs. They review paperwork. And the companies? They cherry-pick data. You think they’d risk a seizure study on 1,200 people? No. They just say ‘bioequivalent’ and move on.
And don’t get me started on ‘inactive ingredients.’ Those are the real toxins. Fillers from China. Dyes from India. You’re not taking medicine-you’re taking a chemistry experiment.
Why do you think so many people get ‘random’ side effects? Because their body is rejecting the plastic, the talc, the cornstarch.
Stick with the brand. Or don’t. But don’t pretend this is science. It’s a scam.
Kristina Williams
November 24, 2025 AT 16:36Did you know the FDA is working with the Illuminati to control your hormones? That’s why they allow the 80-125% range. It’s a mind control experiment.
My cousin’s neighbor’s dog got switched to a generic thyroid med and started barking in Morse code. The vet said it was ‘bioequivalent.’
They’re also hiding the truth about aluminum in the fillers. It’s not just for antiperspirants-it’s in your pills to make you forget you’re being watched.
And why do you think they changed the pill color? So you can’t tell if they swapped your soul.
Check the batch number. If it starts with 7, run.
Shilpi Tiwari
November 25, 2025 AT 17:52From a pharmacokinetic standpoint, the variability in Cmax and AUC within the 80–125% range, while statistically compliant with FDA guidelines, may induce non-linear pharmacodynamic responses in patients with polymorphic CYP450 enzyme expression, particularly for NTI drugs such as warfarin and phenytoin.
Moreover, excipient-induced alterations in gastric transit time and intestinal permeability-mediated by surfactant composition and disintegrant kinetics-can significantly impact bioavailability in subpopulations with altered GI motility, such as elderly patients or those with SIBO.
Therefore, the clinical assertion of ‘interchangeability’ is pharmacologically untenable without therapeutic drug monitoring and individualized bioequivalence profiling.
Authorized generics represent the only viable compromise, as they eliminate inter-manufacturer variability while preserving cost-efficiency.
Future regulatory frameworks must mandate manufacturer-specific bioequivalence labeling, akin to the EU’s ‘code’ system, to prevent inadvertent substitution.
Holly Powell
November 27, 2025 AT 09:19It’s amusing how laypeople romanticize ‘brand’ as some kind of purity. The truth? The brand-name manufacturer often outsources production to the same generic plant. The only difference? A sticker and a $400 markup.
And your ‘lived experience’? Anecdotes aren’t data. You had a bad reaction? Maybe it was stress. Maybe it was diet. Maybe you didn’t take it with food.
Meanwhile, millions of people take generics without issue. Your fear isn’t medical-it’s emotional. And it’s costing the system billions.
Stop romanticizing your pills. They’re not sacred. They’re chemistry.
Emanuel Jalba
November 28, 2025 AT 02:09THIS IS WHY WE CAN’T HAVE NICE THINGS 😭
Somebody’s kid had a seizure because the pharmacy swapped a pill. Someone’s mom got depressed because her thyroid med looked different. And the system just shrugs?
It’s not just about money. It’s about dignity. You deserve to feel normal. You deserve to not wonder if your next pill is going to kill you.
Doctors should be forced to write ‘brand medically necessary’ by default for NTI drugs. No paperwork. No delays. Just… keep people alive.
And if you’re reading this and you’re on one of these meds? You’re not crazy. You’re just smart.
💙 I believe you. I see you. You’re not alone.
Heidi R
November 29, 2025 AT 07:08So you’re telling me I should pay $470 for a pill I can get for $14… because I might feel slightly off?
My insurance won’t cover it. My doctor didn’t even mention it’s a problem.
Guess I’ll just die quietly.